Abstract
INTRODUCTION
Myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML), and acute myeloid leukemia (AML) are rare hematological stem cell disorders resulting in cytopenias, disease-related complications, and death. Given the lack of curative treatment options available to patients with these conditions, patient quality of life is a significant factor when making treatment decisions. The Food and Drug Administration defines health-related quality of life (HRQL) as a multidimensional concept encompassing a patient's overall disease- and treatment-related impact experience associated with physical, psychological (including emotional and cognitive), and social functioning domains. Importantly, regulatory agencies are emphasizing patient-centeredness in clinical trial design to capture benefits of new treatments, and qualitative research is essential to ensure that important aspects of the patients' experiences are captured in pivotal trials. Unfortunately, research on patients' perspectives on their symptoms, treatment and impact on HRQL is limited.
This study aimed to better understand the overall symptom experience and impact on HRQL from the perspective of patients living with higher risk (HR) MDS, CMML, and low blast (LB) AML. Findings informed the development of a conceptual framework (CF) for disease- and treatment-related symptoms and impacts on HRQL.
METHODS
A literature review was conducted in Medline (January 2000-July 2016) for studies reporting on patient symptoms and impacts related to HR MDS, CMML, and LB AML, and supplemented with further medical and advocacy website reviews. Relevant information regarding patients' symptom experience and impact on HRQL was compiled. Semi-structured qualitative interviews with clinicians (n=3) and patients (n=14) elicited further information about core symptoms and related impacts of disease and treatment. Following approval from an independent review board, HR MDS, CMML, and LB AML patients were recruited through the MDS Foundation or physician referral. Patients were screened and compensated. Symptom and impact concepts from all sources were organized into a CF describing the patients' disease and treatment experience and impact on HRQL.
RESULTS
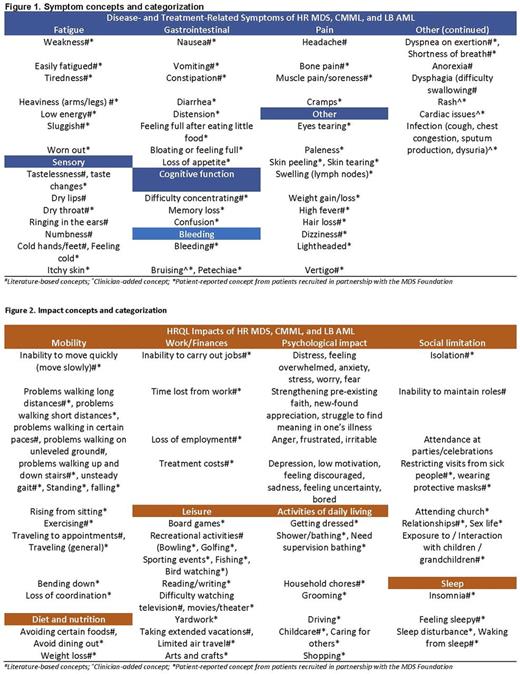
Forty-four disease- and treatment-related symptoms and 49 impacts on HRQL were identified through the literature and website reviews. Clinical experts provided additional background on symptoms of the conditions and common treatment regimens, associated side effects, and potential impact on HRQL. The mean age of patients interviewed was 69 years (±8.5); 64% female; n=11 HR MDS, n=2 CMML, n=1 LB AML. Most patients were treated with azacitidine (n=9; 64%) and date of diagnosis ranged from 4-55 months, with 76% of patients diagnosed within the last 2 years. Eastern Cooperative Oncology Group (ECOG) status was reported by clinicians (n=8; 87% ECOG: 1) or patients (n=6; 83% ECOG: 2). Symptom and impact concepts elicited from patients reflected those identified in the literature review and expert interviews; however, patients provided additional detail that was not reported in other sources. All patients reported fatigue (n=14, 100%); most reported shortness of breath (n=12, 86%), weakness (n=11, 79%), pain (n=10, 64%), nausea (n=9, 64%), bruising (n=9, 64%), constipation (n=9, 64%) and dizziness (n=9, 64%). Symptoms of the disease and treatment had substantial impact on HRQL. Most patients reported difficulty walking (n=11, 79%); performing daily activities such as working (n=9, 64%), household chores (n=8, 57%), shopping (n=7, 50%); and participating in activities that could expose them to infection such as caring for grandchildren (n=7, 50%), traveling (n=6, 43%), and eating out (n=4, 29%). Concepts derived from the literature reviews and clinician and patient interviews were consolidated into two draft CFs (see figures) for disease- and treatment-related symptoms (n=49 concepts) and impacts on HRQL (n=54 concepts).
CONCLUSIONS
Incorporation of the patient voice allowed for direct patient engagement, which provided valuable in-depth insight about the patient experience with HR MDS, CMML and LB AML. The CF for disease- and treatment-related symptoms and impacts on HRQL can guide the evaluation of treatment benefit from the patient perspective in these diseases, which will fill an important gap and help to inform treatment decision making.
Bell: Takeda Pharmaceuticals: Employment, Equity Ownership. Galaznik: Takeda Pharmaceuticals: Employment, Equity Ownership. Pompilus: Modus Outcomes: Employment; Takeda Pharmaceuticals: Research Funding. Strzok: Takeda Pharmaceuticals: Research Funding; Modus Outcomes: Employment. Fram: BeyondSpring Pharmaceuticals, Inc.: Consultancy; Takeda Pharmaceuticals: Consultancy. Faller: Takeda Pharmaceuticals: Employment, Equity Ownership. Marquis: Takeda Pharmaceuticals: Research Funding; Modus Outcomes: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal